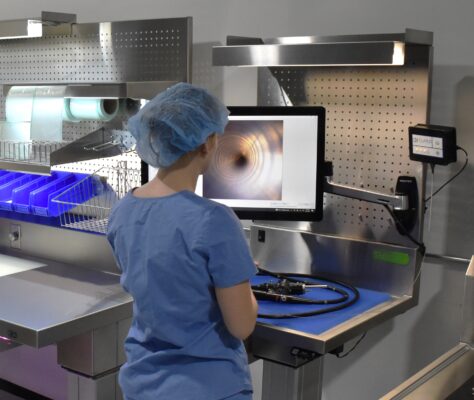
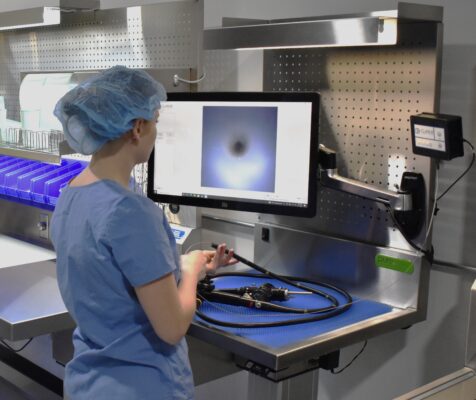
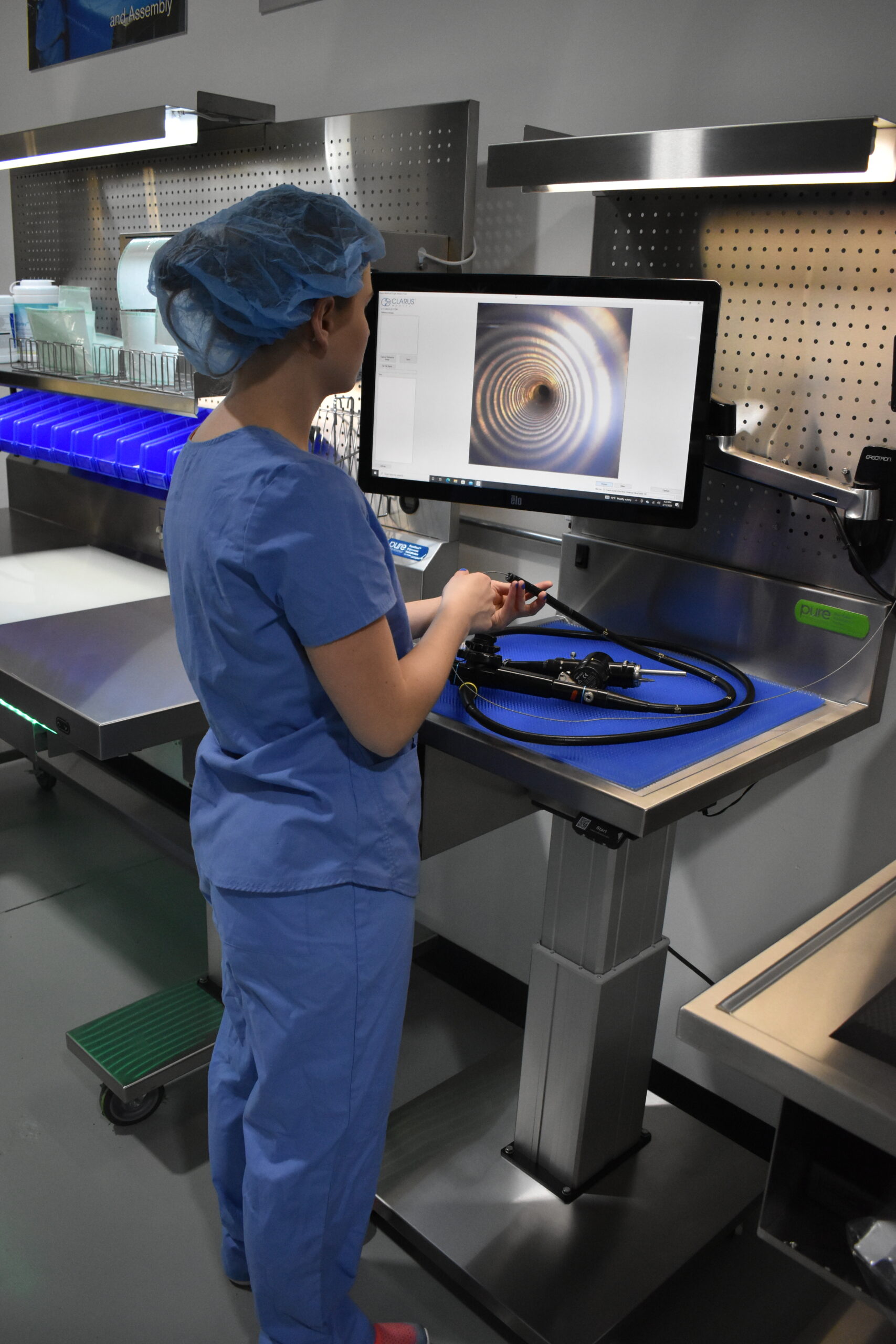
PureClear™ Inspection Scopes allow technicians and nurses to visually inspect internal channel surfaces of lumened instrumentation, including flexible & rigid scopes, shavers, orthopedic, and more. Hard-to-reach areas like elevator channels can be inspected to confirm functional integrity, ensure removal of all debris, and document the cleaning process, helping meet device IFU and national guidelines.
Multiple scope lengths and outer diameters ensure that channels, connection points, distal tips, spaces behind elevator channels, and any small channels can be inspected and documented with video and high-resolution photography.
Documentation includes date, device ID, and time stamps for thorough surveillance.
PureClear Borescopes can be integrated with computer systems, or used with standalone monitors for visual display only.
“…more healthcare-associated outbreaks have been linked to contaminated endoscopes than to any other medical device.” -CDC Guidelines for Disinfection and Sterilization in Healthcare Facilities, 2008
Features to help your SPD and/or GI department
- Inspect channeled instruments for device damage and residual debris
- Provide optional, video and photo documentation of visual inspection
- Removable, waterproof scope catheters allow for easy cleaning and integration into decontamination areas
- Borescope stations only require one control box, but allow for versatility in swapping out different lengths and diameter scope sizes
- Control Modules are small enough for tight spaces, and don't require light sources or cables for use
Technical Specifications
Control Modules (at least 1 is required for any PureClear Inspection Scope set-up)| USB Control Module Options | HDMI Control Module Option |
| Operates on a PC or tablet (required) | No PC required; any standard HDMI monitor (required) |
| Allows for documentation of video & photo, and compatible with most device tracking software | No photo or video capture; requires little IT infrastructure to integrate |
| Scope Length | Outer Diameter | Product ID | Uses |
| 60cm | 1.83mm | CB2-60 | Arthrex shavers, suctions, or other short, wide channels |
| 110cm | 1.83mm | CB2-110 | Most SPD & GI functions |
| 200cm | 1.83mm | CB2-200 | SPD & GI functions; best for the longest channels in your department |
| 110cm | 1.06mm | CB1-110 | Long, thin channels like pediatric scopes; best for the smallest channels requiring visual inspection |